Abstract
Background:
Platelet hyperreactivity involves increased secretion of their granule content which promotes platelet aggregation and thrombosis. Platelet hyperreactivity is observed in conditions such as diabetes mellitus and is associated with decreased cardioprotective effect from antiplatelet agents in this patient group. Diabetes is associated with increased endoplasmic reticulum (ER) stress from hyperglycemia and hyperlipidemia. Protein disulphide isomerase 6 (PDIA6) is an endoplasmic reticulum protein which folds nascent proteins by reduction and oxidation of their disulphide bonds. PDIA6 has been shown to inhibit downstream ER stress pathways by inhibiting the phosphorylation of IRE-1 in fibroblasts (Eletto, Mol Cell, 2014). We hypothesized that ER stress pathways are functional in platelets and that PDIA6 may inhibit ER stress pathways leading to platelet secretion.
Methods:
We generated conditional PDIA6 knockout mice (PF4Cre+ Pdia6 fl/fl) (CKO) in the megakaryocyte/platelet lineage by CRISPR-Cas9 technology (Fig.1A). Megakaryopoiesis and haemostasis was assessed by bone marrow histology, coagulation assays, platelet aggregation and tail bleeding studies. We induced ER stress of purified platelets by incubation with thapsigargin and tunicamycin. Activation of the PERK and IRE1 pathways was measured by Western blot. Thrombosis was assessed in vitro by microfluidic devices and in vivo by electrolytic injury of the carotid artery.
Results:
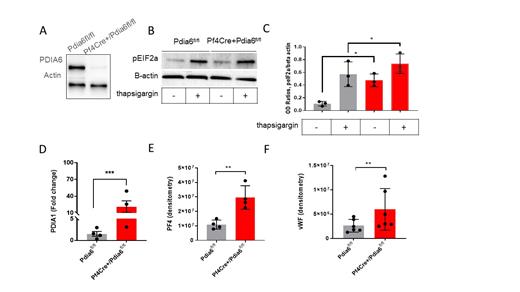
PDIA6 CKO mice displayed a mild macrothrombocytopenia: the mean (+/-SD) platelet count in Pf4Cre+/Pdia6fl/fl was 775 +/- 98 x10 3/ul compared with 874 +/- 55 x10 3/ul in Pdia6fl/fl (p<0.005). The median platelet volume was 6.3 fL in Pf4Cre+/Pdia6fl/fl compared with 5.7 fL in Pdia6fl/fl (p<0.005). Megakaryopoiesis was normal at baseline. However, PDIA6 CKO mice showed significant upregulation of intracellular platelet PDIs including PDIA1, PDIA3 and PDIA4. PDIA6 deficient platelets displayed significant increase of disulphide reductase activity and the generation of free thiols on the platelet surface. Activation of the PERK and IRE-1 pathway at baseline and after induction of ER stress was increased in PDIA6 deficient platelets (Figure 1B, C). There was striking hypersecretion of PDIA1 (Figure 1D) and α-granule proteins (Figure 1E, F) in response to shear and stimulation with thrombin. PDIA6 CKO mice displayed a prothrombotic phenotype with increased platelet adhesion to fibrinogen under shear (500 s-1) and decreased time to carotid artery occlusion (mean+/SD: 10.8 +/-3.2 min in Pf4Cre+/Pdia6fl/fl compared with 15.3 +/-5.2 min in Pdia6fl/fl, n=8-10, p<0.05).
Conclusion:
We have identified a role for platelet PDIA6 in attenuating platelet ER stress and secretion. This opens avenues for further study into the role of platelet PDIs in conditions with increased ER stress, such as obesity and diabetes.
Figure 1: PDIA6 deficient platelets have increased endoplasmic reticulum (ER) stress and are hypersecretory. A. Western blot of PDIA6 protein in platelets from Pf4Cre+/Pdia6fl/fl mice and control mice (Pdia6fl/fl) showing efficient deletion of PDIA6 in platelets. B. PDIA6 deficient platelets have increased phosphorylation of pEIF2a (PERK phosphorylation pathway) at baseline and after induction of ER stress by thapsigargin, representative image. C. Normalized band intensity (peIF2a/beta actin) in platelets treated with DMSO control or thapsigargin. D. Increased secretion of thiol isomerase PDIA1. E. alpha granule proteins: platelet factor 4 (PF4) and F. von Willebrand factor (vWF) from PDIA6 deficient platelets compared with controls after stimulation with thrombin 0.5 U/ml. n=3-5 Pf4Cre+/Pdia6fl/fl (red boxes) and n=3-5 Pdia6fl/fl mice (grey boxes). Columns are presented as mean+/-SD, *p<0.05, ** p<0.001 by Mann Whitney.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal